Cycloaddition reactions between dicyclohexylboron azide and alkynes†
Abstract
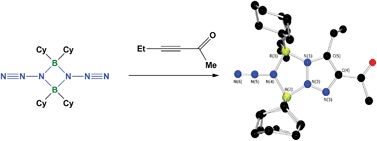
The room temperature 1,3-dipolar cycloaddition reactions of the boron azide, Cy2BN3 with the electron-poor ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2R, EtC
CCO2R, EtC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCOMe and HC
CCOMe and HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CP(
CP(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)Ph2 afforded new
O)Ph2 afforded new ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2R, a new macrocyclic product was isolated with loss of the R group.
CCO2R, a new macrocyclic product was isolated with loss of the R group.

 Please wait while we load your content...
Please wait while we load your content...