Hydrophosphination of vinyl-boranes with phosphinoamines†
Abstract
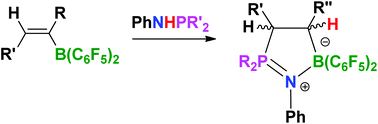
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR2B(C6F5)2 (R1 = Ph, p-FC6H4, nPr, Et, R2 = H, Ph, Et, C6F5) to effect uncatalyzed hydrophosphination of the olefinic bond, affording the C2BNP five-membered cyclization compounds 3–14. All the compounds are fully characterized and compounds 4, 7, 8 and 9 are studied by
CR2B(C6F5)2 (R1 = Ph, p-FC6H4, nPr, Et, R2 = H, Ph, Et, C6F5) to effect uncatalyzed hydrophosphination of the olefinic bond, affording the C2BNP five-membered cyclization compounds 3–14. All the compounds are fully characterized and compounds 4, 7, 8 and 9 are studied by

 Please wait while we load your content...
Please wait while we load your content...