A triangular prismatic hexanuclear iridium(iii) complex bridged by flavin analogues showing reversible redox processes†
Abstract
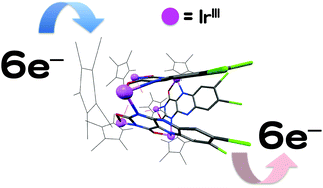
[Ir6(μ-alloCl22−)3(Cp*)6(OH)3](PF6)3 (1) having 7,8-dichloroalloxazine dianion (alloCl22−) as bridging ligands was synthesized and characterized by X-ray crystallography, spectroscopic and electrochemical measurements. The alloxazine ligands showed unprecedented coordination modes to link the six Ir(III) centres. The complex exhibited remarkable stability and reversible six-electron redox processes at the bridging alloxazine ligands in organic solvents. The first reversible reduction process occurred on each of three alloxazine ligands in 1 to produce a three-electron-reduced species, [IrIII6Cp*6(μ-alloCl2˙3−)3(OH)3], and was observed as an apparent one-step reduction process at −0.65 V (vs. Fc0/+). The second reversible reduction process on each of the three alloxazine ligands in 1 was recorded at almost the same potential, −0.78 V (vs. Fc0/+), to afford the six-electron-reduced form, [IrIII6Cp*6(μ-alloCl24−)3(OH)3]3−. The radical anion of the alloxazine derivative was detected by EPR measurements at room temperature. After the six-electron reduction of 1 with cobaltocene, the backward oxidation processes of reduced forms with p-chloranil were traced by UV-Vis spectroscopy to confirm the recovery of the original spectrum of 1.

 Please wait while we load your content...
Please wait while we load your content...