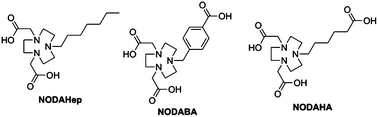
Due to its favorable relaxometric properties, Mn2+ is an appealing metal ion for magnetic resonance imaging (MRI) contrast agents. This paper reports the synthesis and characterization of three new triazadicarboxylate-type ligands and their Mn2+ chelates (NODAHep, 1,4,7-triazacyclononane-1,4-diacetate-7-heptanil; NODABA, 1,4,7-triazacyclononane-1,4-diacetate-7-benzoic acid; and NODAHA, 1,4,7-triazacyclononane-1,4-diacetate-7-hexanoic acid). The protonation constants of the ligands and the stability constants of the chelates formed with Mn2+ and the endogenous Zn2+ ion have been determined by potentiometry. In overall, the thermodynamic stability of the chelates is lower than that of the corresponding NOTA analogues (NOTA = 1,4,7-triazacyclononane-1,4,7-triacetate), consistent with the decreased number of coordinating carboxylate groups. Variable temperature 1H NMRD and 17O NMR measurements have been performed on the paramagnetic chelates to provide information on the water exchange rates and the rotational dynamics. The values of the 17O chemical shifts are consistent with the presence of one water molecule in the first coordination sphere of Mn2+. The three complexes are in the slow to intermediate regime for the water exchange rate, and they all display relatively high rotational correlation times, which explain the relaxivity values between 4.7 and 5.8 mM−1 s−1 (20 MHz and 298 K). These relaxivities are higher than expected for Mn2+ chelates of such size and comparable to those of small monohydrated Gd3+ complexes. The amphiphilic [Mn(NODAHep)] forms micelles above 22 mM (its critical micellar concentration was determined by relaxometry and fluorescence), and interacts with HSA via its alkylic carbon chain providing a 60% relaxivity increase at 20 MHz due to a longer tumbling time.

 Please wait while we load your content...
Please wait while we load your content...