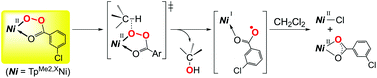
Nickel complexes with hydrotris(pyrazolyl)borate ( = TpR) ligands catalyze alkane oxidation with organic peroxide meta-Cl–C6H4C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OOH ( = mCPBA). The electronic and steric hindrance properties of TpR affect the catalyses. The complex with an electron-withdrawing group containing a less-hindered ligand, that is, TpMe2,Br, exhibits higher alcohol selectivity. Higher selectivity for secondary over tertiary alcohols upon oxidation of methylcyclohexane indicates that the oxygen atom transfer reaction proceeds within the coordination sphere of the nickel centers. A reaction of the catalyst precursor, dinuclear nickel(II)-bis(μ-hydroxo) complexes, with mCPBA yields the corresponding nickel(II)-acylperoxo species, as have been characterized by spectroscopy. Thermal decomposition of the nickel(II)-acylperoxo species in CH2Cl2 yields the corresponding nickel(II)-chlorido complexes through Cl atom abstraction. Employment of the brominated ligand increases the thermal stability of the acylperoxo species. Kinetic isotope effects observed on decay of the nickel(II)-acylperoxo species indicate concerted O–O breaking of the nickel-bound acylperoxide and H-abstraction from the solvent molecule.
O)OOH ( = mCPBA). The electronic and steric hindrance properties of TpR affect the catalyses. The complex with an electron-withdrawing group containing a less-hindered ligand, that is, TpMe2,Br, exhibits higher alcohol selectivity. Higher selectivity for secondary over tertiary alcohols upon oxidation of methylcyclohexane indicates that the oxygen atom transfer reaction proceeds within the coordination sphere of the nickel centers. A reaction of the catalyst precursor, dinuclear nickel(II)-bis(μ-hydroxo) complexes, with mCPBA yields the corresponding nickel(II)-acylperoxo species, as have been characterized by spectroscopy. Thermal decomposition of the nickel(II)-acylperoxo species in CH2Cl2 yields the corresponding nickel(II)-chlorido complexes through Cl atom abstraction. Employment of the brominated ligand increases the thermal stability of the acylperoxo species. Kinetic isotope effects observed on decay of the nickel(II)-acylperoxo species indicate concerted O–O breaking of the nickel-bound acylperoxide and H-abstraction from the solvent molecule.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OOH ( = mCPBA). The electronic and steric hindrance properties of TpR affect the catalyses. The complex with an electron-withdrawing group containing a less-hindered
O)OOH ( = mCPBA). The electronic and steric hindrance properties of TpR affect the catalyses. The complex with an electron-withdrawing group containing a less-hindered 

 Please wait while we load your content...
Please wait while we load your content...