Sandwich-type tetrakis(phthalocyaninato) rare earth(iii)–cadmium(ii) quadruple-deckers. The effect of f-electrons†
Abstract
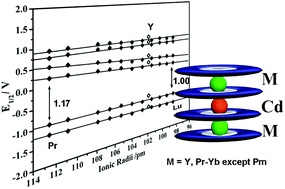
Systematic studies over the synthesis of sandwich-type tetrakis[2,3,9,10,16,17,23,24-octa(octyloxy)phthalocyaninato] heterometallic complexes for the whole series of lanthanide metals and yttrium have been conducted, revealing the dependence of the formation and isolation of the target quadruple-decker compounds on the rare earth ionic size, which is supported by the electronic absorption spectroscopic result. Nevertheless, comparative electrochemical investigations between the lanthanide and yttrium quadruple-deckers reveal the effect of f-electrons, actually the f–f interaction between the lanthanide centers separated by a divalent cadmium ion in the tetrakis(phthalocyaninato) lanthanide–cadmium complexes. The effective imaginary third order molecular hyperpolarizability (Im{χ(3)}) of the whole series of complexes in the range of 2.04–2.41 × 10−11 esu is, however, not ionic size-dependent.

 Please wait while we load your content...
Please wait while we load your content...