Noble metal-free Ni(OH)2–g-C3N4 composite photocatalyst with enhanced visible-light photocatalytic H2-production activity†
Abstract
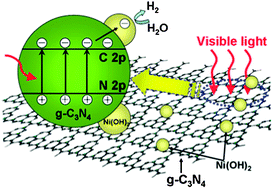
Ni(OH)2-modified graphitic carbon nitride (Ni(OH)2–g-C3N4) composite photocatalysts were prepared by a simple precipitation method using g-C3N4 as support and
- This article is part of the themed collection: Photocatalysis

 Please wait while we load your content...
Please wait while we load your content...