Comparison of methane activation and catalytic ethylene formation on free gold and palladium dimer cations: product binding determines the catalytic turnover
Abstract
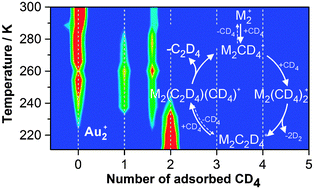
The gas-phase dimers Au2+ and Pd2+ have been shown to readily activate methane and to facilitate its
- This article is part of the themed collection: Gold Catalysis

 Please wait while we load your content...
Please wait while we load your content...