Towards acid MOFs – catalytic performance of sulfonic acid functionalized architectures†
Abstract
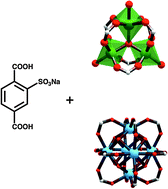
In this work, the inclusion of free sulfonic acid groups in highly stable MOFs is explored. The synthesized
* Corresponding authors
a
Catalysis Engineering, Chemical Engineering Department, Delft University of Technology, Julianalaan 136, Delft, The Netherlands
E-mail:
j.gascon@tudelft.nl
b Institut Lavoisier, UMR 8180 CNRS Université de Versailles St Quentin en Yvelines, 45 avenue des Etats-Unis, 78035 Versailles, France
In this work, the inclusion of free sulfonic acid groups in highly stable MOFs is explored. The synthesized

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
J. Juan-Alcañiz, R. Gielisse, A. B. Lago, E. V. Ramos-Fernandez, P. Serra-Crespo, T. Devic, N. Guillou, C. Serre, F. Kapteijn and J. Gascon, Catal. Sci. Technol., 2013, 3, 2311 DOI: 10.1039/C3CY00272A
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. You can use material from this article in other publications without requesting further permissions from the RSC, provided that the correct acknowledgement is given.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
