Effects of reaction temperature on the photocatalytic activity of photo-SCR of NO with NH3 over a TiO2 photocatalyst†
Abstract
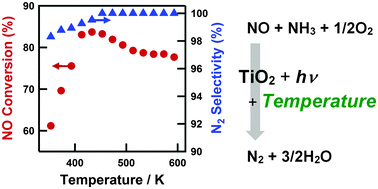
Effects of reaction temperature on the activity of photo-assisted selective catalytic reduction (photo-SCR) of NO with NH3 were investigated under very high GHSV conditions (100 000 h−1). The reaction temperature had a significant effect on the photo-SCR activity over a TiO2 photocatalyst. Maximum NO conversion was achieved at 433 K (NO conversion = 84%, N2 selectivity = 100%). The apparent activation energies in the low and high temperature range were evaluated to be 9.0 kJ mol−1 (353–433 K) and −2.7 kJ mol−1 (493–593 K), respectively. Kinetic analysis revealed that the rate-determining step in the photo-SCR was decomposition of NH2NO intermediates in all the ranges of the reaction temperature (353–593 K). The reaction temperature affects not only the rate constant of decomposition of NH2NO intermediates but also the total number of active sites ([S]0) and the equilibrium constant of NH3 adsorption, resulting in the dynamic change in the reaction rate.
- This article is part of the themed collection: Photocatalysis

 Please wait while we load your content...
Please wait while we load your content...