Zinc-catalyzed Meinwald rearrangement of tetrasubstituted 1-alkynyloxiranes to tertiary α-alkynylketones†
Abstract
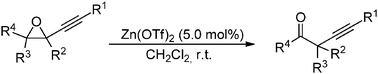
Easy access to tertiary α-alkynylketones via a Meinwald-type rearrangement of 1-alkynyloxiranes is reported. This transformation is selectively accomplished with an economical zinc

 Please wait while we load your content...
Please wait while we load your content...