Palladium catalytic systems with hybrid pyrazole ligands in C–C coupling reactions. Nanoparticles versus molecular complexes†
Abstract
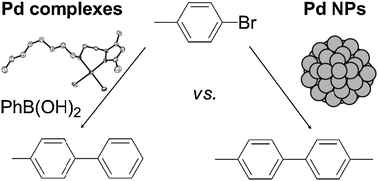
This paper reports the comparison of the chemoselectivity of two different Pd catalytic systems, namely molecular and colloidal systems, in C–C coupling reactions. For this purpose, new hybrid pyrazole derived ligands containing alkylether, alkylthioether or alkylamino moieties have been synthesized and used to form Pd(II) complexes and to stabilize Pd nanoparticles (Pd NPs). With the aim of studying the coordination mode of the ligands and further to understand their role in catalysis, both types of Pd species were characterized by appropriate techniques. In C–C coupling reactions promoted by different Pd colloidal systems, several reports evidenced that active species are molecular catalysts leached from Pd NPs. The most important feature of this work relies on the differences observed in the output of C–C coupling reactions, depending on the colloidal or molecular nature of the catalyst employed. Thus, molecular systems carry out typical Suzuki–Miyaura cross-coupling, together with the dehalogenation of the substrate in different proportions. In contrast, Pd NPs catalyze either Suzuki–Miyaura or C–C homocoupling reactions depending on the haloderivative used. Interestingly, Pd NPs catalyze the quantitative dehalogenation of 4-iodotoluene. Differences observed in the chemoselectivity of these two catalytic systems support that reactions carried out with Pd NPs stabilized with the hybrid pyrazole ligands employed here take place on the surface of the colloids.

 Please wait while we load your content...
Please wait while we load your content...