Thiols, thioethers, and related compounds as sources of C-centred radicals
Abstract
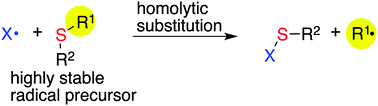
Due to their stability, availability and reactivity, sulfides are particularly attractive sources of carbon-centered radicals. However, their reactivity in homolytic substitution processes is strongly reduced when compared with the corresponding selenides or halides. Despite this, sulfur-containing compounds can be engineered so that they become effective agents in radical chain reactions. A detailed description of the reactivity of organo-sulfur compounds is reported here with the aim of providing clear guidance on the scope and limitation of their use as radical precursors in chain reactions.

 Please wait while we load your content...
Please wait while we load your content...