The modern interpretation of the Wittig reaction mechanism
Abstract
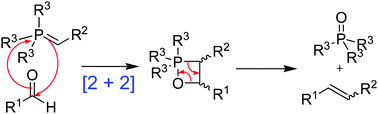
The mechanism of the Wittig reaction has long been a contentious issue in organic chemistry. Even now, more than 50 years after its announcement, its presentation in many modern undergraduate textbooks is either overly simplified or entirely inaccurate. In this review, we gather together the huge body of evidence that has been amassed to show that the Li salt-free Wittig reactions of non-stabilised, semi-stabilised and stabilised ylides all occur under kinetic control by a common mechanism in which oxaphosphetane (OPA) is the first-formed and only intermediate. The numerous recent significant additions to the subject – including computational studies and experimental material pertinent to both steps of the reaction (OPA formation and its decomposition) are discussed in detail, and the currently accepted explanations for the source of the stereoselectivity in Wittig reactions are given. We also present the other mechanistic proposals that have been made during the history of the Wittig reaction, and show how they are unable to account for all of the experimental evidence that is now available. Details of certain experimental facts to do with Wittig reactions in the presence of Li cation are also included, although the precise mechanistic details of such reactions are yet to be established conclusively. We make the case that a clear distinction should henceforth be made between the unknown “Li-present” and the now well-established “Li salt-free” Wittig mechanisms.

 Please wait while we load your content...
Please wait while we load your content...