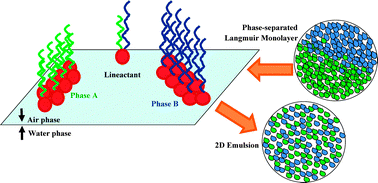
In 1861 Thomas Graham gave birth to a new field of science, today known as colloid science. Nowadays, the notion “colloid” is often used referring to systems consisting of two immiscible phases, one of which is finely dispersed into the other. Research on colloids deals mostly with sols (solids dispersed in a liquid), emulsions (liquids dispersed in liquid), and foams (gas dispersed in a liquid). Because the dispersed particles are small, there is a lot of interface per unit mass. Not surprisingly, therefore, the properties of the interface have often a decisive effect on the behaviour of colloids. Water–air interfaces have a special relevance in this field: many water-insoluble molecules can be spread on water and, given the right spreading conditions and enough available surface area, their spreading proceeds until a monolayer (a one-molecule thick layer) eventually remains. Several 2D phases have been identified for such monolayers, like “gas”, “liquid expanded”, “liquid condensed”, and “solid”. The central question of this review is whether these 2D phases can also exist as colloidal systems, and what stabilizes the dispersed state in such systems. We shall present several systems capable of yielding 2D phase separation, from those based on either natural or fluorinated amphiphiles, to polymer-based ones. We shall seek for analogies in 3D and we shall try to clarify if the lines between these 2D objects play a similar role as the interfaces between 3D colloidal systems. In particular, we shall consider the special role of molecules that tend to accumulate at the phase boundaries, that is, at the contact lines, which will therefore be denoted “line-actants” (molecules that adsorb at a 1D interface, separating two 2D colloidal entities), by analogy to the term “surfactant” (which indicates a molecule that adsorbs at a 2D interface separating two 3D colloidal entities).

 Please wait while we load your content...
Please wait while we load your content...