Large amplitude motion in cold monohydrated dihydrogen phosphate anions H2PO4−(H2O): infrared photodissociation spectroscopy combined with ab initio molecular dynamics simulations†
Abstract
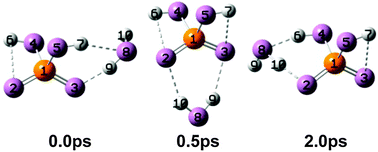
The vibrational spectroscopy of monohydrated dihydrogen phosphate anions, H2PO4−(H2O), is studied in the O–H stretching (2700–3900 cm−1) and the fingerprint regions (600–1800 cm−1). Assignment of the experimental infrared multiple photon photodissociation spectra based on the predicted harmonic spectra of energetically low-lying 0 K structures is not conclusive. Ab initio molecular dynamics simulations reveal that the water molecule undergoes large amplitude motion, even at low internal temperatures, and that the dipole time correlation function qualitatively captures the anharmonic effects of the low-barrier isomerization reaction on the infrared intensities.

 Please wait while we load your content...
Please wait while we load your content...