In situ generation of a hydroxyl radical by nanoporous activated carbon derived from rice husk for environmental applications: kinetic and thermodynamic constants†
Abstract
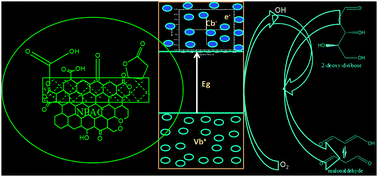
The objective of this investigation is to evaluate the hydroxyl radical (˙OH) generation using nanoporous activated carbon (NPAC), derived from rice husk, and dissolved oxygen in water. The in situ production of the ˙OH radical was confirmed through the DMPO spin trapping method in EPR spectroscopy and quantitative determination by a deoxyribose assay procedure. NPAC served as a heterogeneous catalyst to degrade 2-deoxy-D-ribose (a reference compound) using hydroxyl radical generated from dissolved oxygen in water at temperatures in the range 313–373 K and pH 6, with first order rate constants (k = 9.2 × 10−2 min−1, k = 1.2 × 10−1 min−1, k = 1.3 × 10−1 min−1 and k = 1.68 × 10−1 min−1). The thermodynamic constants for the generation of hydroxyl radicals by NPAC and dissolved oxygen in water were ΔG −1.36 kJ mol−1 at 313 K, ΔH 17.73 kJ mol−1 and ΔS 61.01 J mol−1 K−1.

 Please wait while we load your content...
Please wait while we load your content...