Simulated swelling during low-temperature N2 adsorption in polymers of intrinsic microporosity†
Abstract
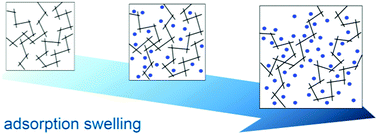
Polymers of intrinsic microporosity (PIMs) are a promising alternative in gas separation applications; however, their industrial applicability is greatly reduced by their susceptibility toward sorption-induced plasticization. In this study, the effects of swelling during nitrogen adsorption isotherms at 77 K for five PIMs (PIM-1, PIM-1c, PIM-SBF, PIM-SBF-Me, and sPIM-1) were examined through predictive molecular simulations. The structural changes and adsorptive implications of swelling each PIM from 0 to 15% were analyzed by generating swollen “snapshots” of the PIM during the swelling process by means of an effective swelling procedure. The size of the free volume elements increased with the simulated swelling percentage, while the closely packed polymer chains remained associated. By comparing the simulated swollen and available experimental isotherms, this investigation shows that the amount of swelling observed during low-temperature nitrogen adsorption is strongly correlated with the interaction strength of the polymer matrix. Therefore, this study provides further support that PIMs should be designed with functionalities to increase the associative interactions of adjacent polymer chains while simultaneously prohibiting efficient packing to construct a gas separation membrane with limited low-temperature N2 swelling and high free volumes.

 Please wait while we load your content...
Please wait while we load your content...