Influence of the tyrosine environment on the second harmonic generation of iturinic antimicrobial lipopeptides at the air–water interface
Abstract
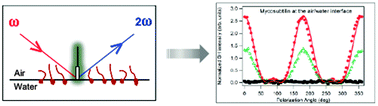
The second harmonic generation (SHG) response at the air–water interface from the tyrosine-containing natural iturinic cyclo-lipopeptides mycosubtilin, iturin A and bacillomycin D is reported. It is shown that this response is dominated by the single tyrosine residue present in these molecules owing to the large first hyperpolarizability arising from the non-centrosymmetric aromatic ring structure of this amino acid. The SHG response of these iturinic antibiotics is also compared to the response of surfactin, a cyclo-lipopeptide with a similar L,D-amino acid sequence but lacking a tyrosine residue, and PalmATA, a synthetic linear lipopeptide possessing a single tyrosine residue but lacking the amino acid sequence structuring the cycle of the iturinic antibiotics. From the light polarization analysis of the SHG response, it is shown that the tyrosine local environment is critical in defining the SHG response of these peptides at the air–water interface. Our results demonstrate that tyrosine, similar to tryptophan, can be used as an endogenous molecular probe of peptides and proteins for SHG at the air–water interface, paving the way for SHG studies of other tyrosine-containing bioactive molecules.

 Please wait while we load your content...
Please wait while we load your content...