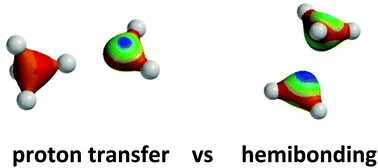
The basin hopping search algorithm in conjunction with second-order Møller–Plesset perturbation theory is used to determine the lowest energy structures of the radical cation clusters (NH3)n˙+, (H2O)n˙+, (HF)n˙+, (PH3)n˙+, (H2S)n˙+ and (HCl)n˙+, where n = 2–4. The energies of the most stable structures are subsequently evaluated using coupled cluster theory in conjunction with the aug-cc-pVTZ basis set. These cationic clusters can adopt two distinct structural types, with some clusters showing an unusual type of bonding, often referred to as hemibonding, while other clusters undergo proton transfer to give an ion and radical. It is found that proton transfer based structures are preferred by the (NH3)n˙+, (H2O)n˙+ and (HF)n˙+ clusters while hemibonded structures are favoured by (PH3)n˙+, (H2S)n˙+ and (HCl)n˙+. These trends can be attributed to the relative strengths of the molecules and molecular cations as Brønsted bases and acids, respectively, and the strength of the interaction between the ion and radical in the ion–radical clusters.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...