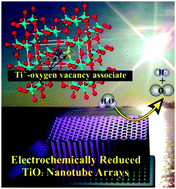
Hetero-element doping (e.g., N, F, C) of TiO2 is inevitably accompanied by significantly increased structural defects due to the dopants' nature being foreign impurities. Very recently, in situ self-doping with homo-species (e.g., Ti3+) has been emerging as a rational solution to enhance TiO2 photoactivity within both UV and visible light regions. Herein we demonstrate that conventional electrochemical reduction is indeed a facile and effective strategy to induce in situ self-doping of Ti3+ into TiO2 and the self-doped TiO2 photoelectrodes showed remarkably improved and very stable water splitting performance. In this study, hierarchical TiO2 nanotube arrays (TiO2 NTs) were chosen as TiO2 substrates and then electrochemically reduced under varying conditions to produce Ti3+ self-doped TiO2 NTs (ECR-TiO2 NTs). The optimized saturation photocurrent density and photoconversion efficiency on the ECR-TiO2 NTs under simulated AM 1.5G illumination were identified to be 2.8 mA cm−2 at 1.23 V vs. RHE and 1.27% respectively, which are the highest values ever reported for TiO2 based photoelectrodes. The electrochemical impedance spectra measurement confirms that the electrochemical induced Ti3+ self-doping improved the electrical conductivity of the ECR-TiO2 NTs. The versatility and effectiveness of the electrochemical reduction method for Ti3+ self-doping in P25 based TiO2 was also examined and confirmed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...