Mechanisms of the ammonia oxidation by hydrogen peroxide over the perfect and defective Ti species of TS-1 zeolite†
Abstract
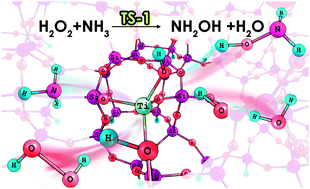
The catalytic performances of titanium species in TS-1 zeolite for the hydroxylamine formation have been investigated using the density functional theory with the ONIOM scheme. The reaction process for making hydroxylamine is divided into two steps: (i) the H2O2 decomposition over the Ti species to produce the peroxo titanium species and (ii) the NH3 oxidation over the generated oxidizing species. Our results indicated that defective Ti species in the TS-1 zeolite are the dominant catalytic sites for H2O2 decomposition rather than perfect Ti species, leading to the formation of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ti–OOH species as oxygen-donating intermediates for NH3 oxidation reaction. The energetic profiles for the ammonia oxidation over the
Ti–OOH species as oxygen-donating intermediates for NH3 oxidation reaction. The energetic profiles for the ammonia oxidation over the ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) TiOOH species and the catalytic effect from water were fully investigated, consisting of three proposed mechanisms. The most favored pathway was found to be: the adsorption of ammonia (NH3/η1
TiOOH species and the catalytic effect from water were fully investigated, consisting of three proposed mechanisms. The most favored pathway was found to be: the adsorption of ammonia (NH3/η1![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) TiOOH) → ammonia oxide complex (NH3O/
TiOOH) → ammonia oxide complex (NH3O/![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) TiOH) → hydrated-titanium-oxyamine species (H2O/
TiOH) → hydrated-titanium-oxyamine species (H2O/![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) TiONH2) → hydroxylamine product (NH2OH/
TiONH2) → hydroxylamine product (NH2OH/![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) TiOH), in which the highest energy barrier is 16.3 kcal mol−1. Besides the hydrolysis of titanium-oxyamine species, the hydroxylamine was also generated through the second H2O2 decomposition over the titanium-oxyamine species whereas the activation energy for this step was slightly decreased to be 15.7 kcal mol−1.
TiOH), in which the highest energy barrier is 16.3 kcal mol−1. Besides the hydrolysis of titanium-oxyamine species, the hydroxylamine was also generated through the second H2O2 decomposition over the titanium-oxyamine species whereas the activation energy for this step was slightly decreased to be 15.7 kcal mol−1.

 Please wait while we load your content...
Please wait while we load your content...