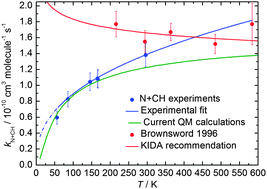
Rate constants for the potentially important interstellar N(4S) + CH(X2Πr) reaction have been measured in a continuous supersonic flow reactor over the range 56 K ≤ T ≤ 296 K using the relative rate technique employing both the N(4S) + OH(X2Πi) and N(4S) + CN(X2Σ+) reactions as references. Excess concentrations of atomic nitrogen were produced by the microwave discharge method upstream of the Laval nozzle and CH and OH radicals were created by the in situ pulsed laser photolysis of suitable precursor molecules. In parallel, quantum dynamics calculations of the title reaction have been performed based on accurate global potential energy surfaces for the 13A′ and 13A′′ states of HCN and HNC, brought about through a hierarchical construction scheme. Both adiabatic potential energy surfaces are barrierless, each one having two deep potential wells suggesting that this reaction is dominated by a complex-forming mechanism. The experimental and theoretical work are in excellent agreement, predicting a positive temperature dependence of the rate constant, in contrast to earlier experimental work at low temperature. The effects of the new low temperature rate constants on interstellar N2 formation are tested using a dense cloud model, yielding N2 abundances 10–20% lower than previously predicted.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...