Relativistic effects on acidities and basicities of Brønsted acids and bases containing gold
Abstract
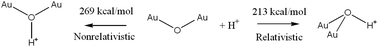
It is usually believed that relativistic effects as described by the Dirac–Schrödinger equation (relative to the classical or time-independent Schrödinger equation) are of little importance in chemistry. A closer look, however, reveals that some important and widely known properties (e.g., gold is yellow, mercury is liquid at room temperature) stem from relativistic effects. So far the influence of relativistic effects on the acid–base properties has been mostly ignored. Here we show that at least for compounds of gold such omission is completely erroneous and would lead to too high basicity and too low acidity values with errors in the range of 25–55 kcal mol−1 (or 20 to 44 powers of ten in pKa units) in the gas-phase. These findings have important implications for the design of new superstrong acids and bases, and for the understanding of gold-catalysed reactions.

 Please wait while we load your content...
Please wait while we load your content...