Conformational control of benzophenone-sensitized charge transfer in dinucleotides†
Abstract
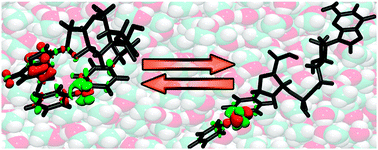
Charge transfer in DNA cannot be understood without addressing the complex conformational flexibility, which occurs on a wide range of timescales. In order to reduce this complexity four dinucleotide models 1X consisting of benzophenone linked by a phosphodiester to one of the natural nucleosides X = A, G, T, C were studied in water and methanol. The theoretical work focuses on the dynamics and electronic structure of 1G. Predominant conformations in the two solvents were obtained by molecular dynamics simulations. 1G in MeOH adopts mainly an open geometry with a distance of 12–16 Å between the two aromatic parts. In H2O the two parts of 1G form primarily a stacked conformation yielding a distance of 5–6 Å. The low-lying excited states were investigated by electronic structure theory in a QM/MM environment for representative snapshots of the trajectories. Photo-induced intramolecular charge transfer in the S1 state occurs exclusively in the stacked conformation. Ultrafast transient absorption spectroscopy with 1X reveals fast charge transfer from S1 in both solvents with varying yields. Significant charge transfer from the T1 state is only found for the nucleobases with the lowest oxidation potential: in H2O, charge transfer occurs with 3.2 × 109 s−1 for 1A and 6.0 × 109 s−1 for 1G. The reorganization energy remains nearly unchanged going from MeOH to the more polar H2O. The electronic coupling is rather low even for the stacked conformation with HAB = 3 meV and explains the moderate charge transfer rates. The solvent controls the conformational distribution and therefore gates the charge transfer due to differences in distance and stacking.

 Please wait while we load your content...
Please wait while we load your content...