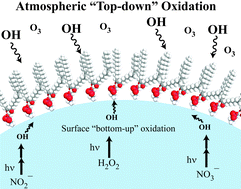
Biological organic compounds mixed with NaCl and other inorganic compounds in sea-salt aerosol particles react in air with oxidants such as ozone and hydroxyl (OH) radicals. Laboratory studies of model systems can provide insight into these reactions. We report here studies of the kinetics and mechanism of oxidation of unsaturated 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) on NaCl by gas phase OH in air at room temperature and 1 atm pressure using diffuse reflection infrared Fourier transform spectrometry (DRIFTS) and matrix-assisted laser desorption/ionization-time-of-flight-mass spectrometry (MALDI-TOF-MS) to identify possible structures of surface-bound reaction products. For comparison, some studies were also carried out on the saturated 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) on NaCl. The calculated concentration of hydroxyl radicals, generated by photolysis of isopropyl nitrite, was (1.6–6.4) × 108 radicals cm−3. Surface-bound aldehydes, ketones, organic nitrates and nitrate ions were identified as products of these reactions and structures of potential products were proposed based on mechanistic considerations combined with the MALDI-TOF-MS and DRIFTS spectra. The loss rate of vinyl hydrogen, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H, at 3008 cm−1 was used to obtain a lower limit for the rate constant (k1) for addition of OH to the double bond, k1 > (3 ± 1) × 10−13 cm3 molecule−1 s−1 (1s), corresponding to a reaction probability of γadd > (4 ± 1) × 10−3 (1s). Assuming that abstraction from –CH2– groups in POPC is the same as for DPPC, the percentage of the reaction that occurs by addition is ∼80%. This is similar to the percent addition predicted using structure–reactivity relationships for gas-phase reactions. Decreasing the amount of POPC relative to NaCl resulted in more nitrate ion formation and less relative loss of POPC, and increasing the OH concentration resulted in a more rapid loss of POPC and faster product formation. These studies suggest that under atmospheric conditions with an OH concentration of 5 × 106 radicals cm−3, the lifetime of POPC with respect to OH is <6 days.
C–H, at 3008 cm−1 was used to obtain a lower limit for the rate constant (k1) for addition of OH to the double bond, k1 > (3 ± 1) × 10−13 cm3 molecule−1 s−1 (1s), corresponding to a reaction probability of γadd > (4 ± 1) × 10−3 (1s). Assuming that abstraction from –CH2– groups in POPC is the same as for DPPC, the percentage of the reaction that occurs by addition is ∼80%. This is similar to the percent addition predicted using structure–reactivity relationships for gas-phase reactions. Decreasing the amount of POPC relative to NaCl resulted in more nitrate ion formation and less relative loss of POPC, and increasing the OH concentration resulted in a more rapid loss of POPC and faster product formation. These studies suggest that under atmospheric conditions with an OH concentration of 5 × 106 radicals cm−3, the lifetime of POPC with respect to OH is <6 days.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H, at 3008 cm−1 was used to obtain a lower limit for the rate constant (k1) for addition of OH to the double bond, k1 > (3 ± 1) × 10−13 cm3 molecule−1 s−1 (1s), corresponding to a reaction probability of γadd > (4 ± 1) × 10−3 (1s). Assuming that abstraction from –CH2– groups in POPC is the same as for DPPC, the percentage of the reaction that occurs by addition is ∼80%. This is similar to the percent addition predicted using structure–reactivity relationships for gas-phase reactions. Decreasing the amount of POPC relative to NaCl resulted in more nitrate ion formation and less relative loss of POPC, and increasing the OH concentration resulted in a more rapid loss of POPC and faster product formation. These studies suggest that under atmospheric conditions with an OH concentration of 5 × 106 radicals cm−3, the lifetime of POPC with respect to OH is <6 days.
C–H, at 3008 cm−1 was used to obtain a lower limit for the rate constant (k1) for addition of OH to the double bond, k1 > (3 ± 1) × 10−13 cm3 molecule−1 s−1 (1s), corresponding to a reaction probability of γadd > (4 ± 1) × 10−3 (1s). Assuming that abstraction from –CH2– groups in POPC is the same as for DPPC, the percentage of the reaction that occurs by addition is ∼80%. This is similar to the percent addition predicted using structure–reactivity relationships for gas-phase reactions. Decreasing the amount of POPC relative to NaCl resulted in more nitrate ion formation and less relative loss of POPC, and increasing the OH concentration resulted in a more rapid loss of POPC and faster product formation. These studies suggest that under atmospheric conditions with an OH concentration of 5 × 106 radicals cm−3, the lifetime of POPC with respect to OH is <6 days.

 Please wait while we load your content...
Please wait while we load your content...