Effect of water and ammonia on surface species formed during NOx storage–reduction cycles over Pt–K/Al2O3 and Pt–Ba/Al2O3 catalysts
Abstract
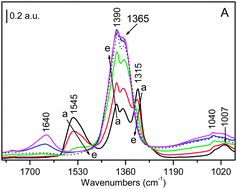
The effect of water, in the temperature range 25–350 °C, and ammonia at RT on two different surface species formed on Pt–K/Al2O3 and Pt–Ba/Al2O3 NSR catalysts during NOx storage–reduction cycles was investigated. The surface species involved are nitrates, formed during the NOx storage step, and isocyanates, which are found to be intermediates in N2 production during reduction by CO. FT-IR experiments demonstrate that the dissociative chemisorption of water and ammonia causes the transformation of the bidentate nitrates and linearly bonded NCO− species into more symmetric species that we call ionic species. In the case of water, the effect on nitrates is observable at all the temperatures studied; however, the extent of the transformation decreases upon increasing temperature, consistent with the decreased extent of dissociatively adsorbed water. It was possible to hypothesize that the dissociative chemisorption of water and ammonia takes place in a competitive way on surface sites able to give bidentate nitrates and linearly bonded NCO− that are dislocated, remaining on the surface as ionic species.
- This article is part of the themed collection: Physical chemistry at the cross-road of advanced oxide materials

 Please wait while we load your content...
Please wait while we load your content...