Amyloid-β–neuropeptide interactions assessed by ion mobility-mass spectrometry
Abstract
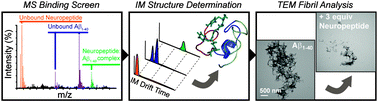
Recently, small peptides have been shown to modulate aggregation and toxicity of the amyloid-β protein (Aβ). As such, these new scaffolds may help discover a new class of biotherapeutics useful in the treatment of Alzheimer's disease. Many of these inhibitory peptide sequences have been derived from natural sources or from Aβ itself (e.g., C-terminal Aβ fragments). In addition, much earlier work indicates that tachykinins, a broad class of neuropeptides, display neurotrophic properties, presumably through direct interactions with either Aβ or its receptors. Based on this work, we undertook a limited screen of neuropeptides using ion mobility-mass spectrometry to search for similar such peptides with direct Aβ binding properties. Our results reveal that the neuropeptides leucine enkephalin (LE) and galanin interact with both the monomeric and small oligomeric forms of Aβ1–40 to create a range of complexes having diverse stoichiometries, while some tachyknins (i.e., substance P) do not. LE interacts with Aβ more strongly than galanin, and we utilized ion mobility-mass spectrometry, molecular dynamics simulations, gel electrophoresis/Western blot, and transmission electron microscopy to study the influence of this peptide on the structure of Aβ monomer, small Aβ oligomers, as well as the eventual formation of Aβ fibrils. We find that LE binds selectively within a region of Aβ between its N-terminal tail and hydrophobic core. Furthermore, our data indicate that LE modulates fibril generation, producing shorter fibrillar aggregates when added in stoichiometric excess relative to Aβ.
- This article is part of the themed collection: Biophysical studies on protein misfolding and amyloid diseases

 Please wait while we load your content...
Please wait while we load your content...