Solvent states and spectroscopy of doped helium clusters as a quantum-chemistry-like problem†
Abstract
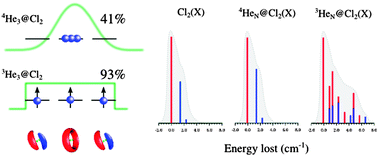
The Full-Configuration-Interaction Nuclear-Orbital (FCI-NO) approach [J. Chem. Phys., 2009, 131, 19401], as the implementation of the quantum-chemistry ansatz, is overviewed and applied to (He)N–Cl2(X) clusters (N ≤ 4). The ground and excited states of both fermionic 3He and bosonic 4He [see also, J. Phys. Chem. Lett., 2012, 2, 2145] clusters are studied. It is shown that the FCI-NO approach allows us to overcome three main difficulties: (1) the Fermi–Dirac (Bose–Einstein) nuclear statistics; (2) the wide (highly anharmonic) amplitudes of the He–dopant and He–He motions; and (3) both the weakly attractive (long-range) and the strongly repulsive (short-range) interaction between the helium atoms. Special emphasis is placed on the dependence of the cluster properties on the number of helium atoms, and on the comparison between the two helium isotopes. In particular, we analyze the analogies between quantum rings comprising electrons and 3He atoms. The synthetic vibro-rotational
- This article is part of the themed collection: Spectroscopy and dynamics of medium-sized molecules and clusters

 Please wait while we load your content...
Please wait while we load your content...