Electron spin resonance studies of trityl OX063 at a concentration optimal for DNP
Abstract
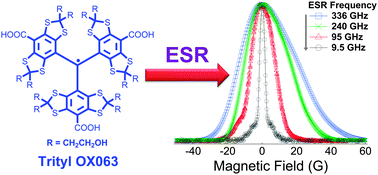
We have performed temperature-dependent electron spin resonance (
* Corresponding authors
a
Advanced Imaging Research Center, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, Texas 75390, USA
E-mail:
matthew.merritt@utsouthwestern.edu
Fax: +1-214-645-2744
Tel: +1-214-645-2750
b Department of Chemistry, University of Texas at Dallas, 800 West Campbell Road, Richardson, Texas 75080, USA
c Molecular Biophysics, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, Texas 75390, USA
d VA North Texas Healthcare System, Dallas, TX 75216, USA
e Department of Physics, Florida State University, 77 Chieftan Way, Tallahassee, FL 32306, USA
f National High Magnetic Field Laboratory, Florida State University, 1800 East Paul Dirac Drive, Tallahassee, Florida 32310, USA
g Biomedical Engineering, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, Texas 75390, USA
h Department of Bioengineering, University of Texas at Dallas, 800 West Campbell Road, Richardson, Texas 75080, USA
We have performed temperature-dependent electron spin resonance (

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
L. Lumata, Z. Kovacs, A. D. Sherry, C. Malloy, S. Hill, J. van Tol, L. Yu, L. Song and M. E. Merritt, Phys. Chem. Chem. Phys., 2013, 15, 9800 DOI: 10.1039/C3CP50186H
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
