Effects of hydrogen dissociation on the infrared emission spectra of naphthalene: theoretical modeling†
Abstract
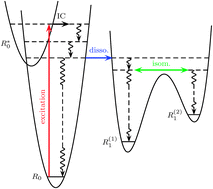
The IR emission
- This article is part of the themed collection: Spectroscopy and dynamics of medium-sized molecules and clusters

 Please wait while we load your content...
Please wait while we load your content...