Hydrogen bonding involving side chain exchangeable groups stabilizes amyloid quarternary structure
Abstract
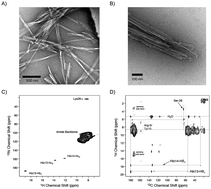
The amyloid β-peptide (Aβ) is the major structural component of amyloid fibrils in the plaques of brains of Alzheimer's disease patients. Numerous studies have addressed important aspects of secondary and tertiary structure of fibrils. In electron microscopic images, fibrils often bundle together. The mechanisms which drive the association of protofilaments into bundles of fibrils are not known. We show here that amino acid side chain exchangeable groups like e.g. histidines can provide useful restraints to determine the quarternary assembly of an amyloid fibril. Exchangeable protons are only observable if a side chain hydrogen bond is formed and the respective protons are protected from exchange. The method relies on deuteration of the Aβ peptide. Exchangeable deuterons are substituted with protons, before fibril formation is initiated.
- This article is part of the themed collection: Biophysical studies on protein misfolding and amyloid diseases

 Please wait while we load your content...
Please wait while we load your content...