Infrared photodissociation spectra of mass selected homoleptic nickel carbonyl cluster cations in the gas phase†
Abstract
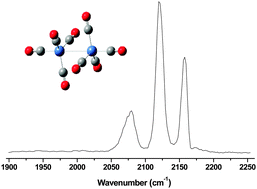
- This article is part of the themed collection: Spectroscopy and dynamics of medium-sized molecules and clusters

 Please wait while we load your content...
Please wait while we load your content...