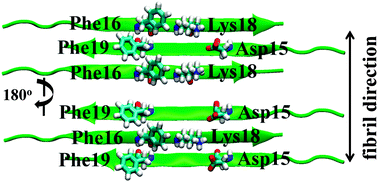
Calcitonin (CT) is an amyloid fibril forming peptide. Since salmon calcitonin (sCT), having Leu residues (Leu12, Leu16 or Leu19) instead of Tyr12, Phe16 or Phe19 for human calcitonin (hCT), is known to form the fibrils much slower than hCT, hCTs mutated to Leu residues at the position of 16 (F16L-hCT), 19 (F19L-hCT), and 12, 16 and 19 (TL-hCT) were examined to reveal the role of aromatic side-chains on amyloid fibrillation using solid-state 13C NMR. The detailed kinetics were analyzed using a two-step reaction mechanism such as nucleation and fibril elongation with the rate constants of k1 and k2, respectively. The k2 values of hCT mutants were significantly slower than that of hCT at a neutral pH, although they were almost the same at an acidic pH. The 13C chemical shifts of the labeled sites showed that the conformations of monomeric hCT mutants take α-helices as viewed from the Gly10 moiety. The hCT mutants formed fibrils and during the fibril formation, the α-helix around Gly10-Phe22 changed to the β-sheet, and the major structures around Ala26-Ala31 were random coil in the fibrils. Molecular dynamics simulation was performed for the β-sheet system of hCT9-23 and its mutants F16L-hCT9-23, F19L-hCT9-23 and TL-hCT9-23. In one of the stable fibril structures, Phe16 of hCT interacts with Phe19 of the next strand alternatively. In the hCT mutants, lack of Phe16 and Phe19 interaction causes significant instability as compared with the hCT fibril, leading to the reduction of k2 values, as observed experimentally in the hCT mutants at a neutral pH.

 Please wait while we load your content...
Please wait while we load your content...