Hysteresis and the role of nucleation and growth in the hydrogenation of Mg nanolayers†
Abstract
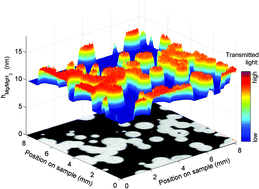
We investigated the hydrogenation of 3 and 10 nm Mg layers sandwiched between Ti using an optical transmission technique (hydrogenography). We observe in situ the two dimensional nucleation and growth of single

 Please wait while we load your content...
Please wait while we load your content...