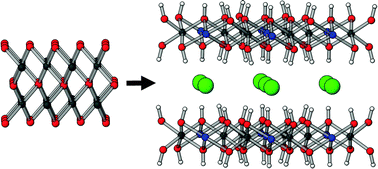
The formation of the layered double hydroxide [Cu2Cr(OH)6]Cl·yH2O from the reaction between CuO and aqueous CrCl3·6H2O was explored using synchrotron X-ray diffraction and ex situ analyses. The use of hard X-rays permitted time-resolved in situ studies to be performed as the reaction proceeded under a range of conditions. Additional information was obtained from ex situ experiments in which aliquots of the reaction mixture were removed, quenched, and subsequently analysed by laboratory X-ray diffraction, IR, UV-visible, and atomic emission spectroscopies. On the basis of these data, it is proposed that the reaction involves three steps. First, the solid CuO starting material is hydrolysed to give Cu(OH)2 chains, releasing Cu2+ ions into solution. The Cu hydroxide chains subsequently condense with aqueous Cr3+ species, Cl− ions and water molecules to give a hydrated form of the LDH. This material then extrudes some water to form a phase with a reduced interlayer spacing.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...