Time-resolved kinetic studies of the reaction of dimethylsilylene, SiMe2, generated by laser flash photolysis of 1,1-dimethyl-1-silacyclopent-3-ene, have been carried out to obtain rate coefficients for its bimolecular reactions with trimethylsilane-1-d, Me3SiD. The reaction was studied in the gas phase at five temperatures in the range 292–605 K. The rate coefficients showed no pressure dependence in the presence of up to 13 kPa of SF6. The second order rate coefficients obtained at 0.7 kPa fitted the Arrhenius equation: log(k/cm3 molecule−1 s−1) = (−13.53 ± 0.19) + (11.29 ± 1.46) kJ mol−1/RT ln 10. By comparison with rate coefficients obtained previously for the reaction of SiMe2 with Me3SiH, a set of kinetic isotope effects, kH/kD, of value ca. 1.2 showing very little temperature dependence was obtained. Theoretical support for these values has been obtained by means of quantum chemical calculations used in conjunction with transition state theory. This study provides the first comprehensive set of kinetic isotope effects for the Si–H(D) insertion process of a silylene in the gas phase.
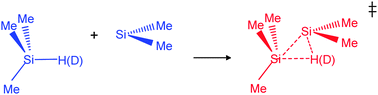
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
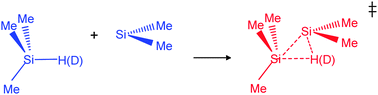

 Please wait while we load your content...
Please wait while we load your content...