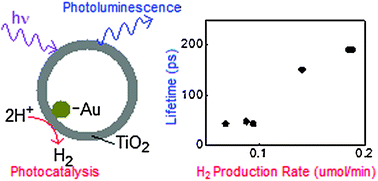
The spectroscopic and photocatalytic properties of a series of Au@TiO2 core–shell nanostructures are characterized. The crystallinity of the TiO2 shells was varied by changing the etching and calcination conditions. Measurements of the photoluminescence, transient absorption, and H2 production rate permit us to look for correlations between the spectroscopic and catalytic behaviors. We found that there is a strong effect of crystallinity on the H2 production rate and also the stretched exponential lifetime of the photoluminescence created by short-wavelength (266 and 300 nm) photoexcitation. As the TiO2 crystallinity is increased, the photoluminescence lifetime increases from 22 to 140 ps in a 1 ns detection window, while the H2 production rate increases by a factor of ∼4. There is no discernible effect of crystallinity on the photoluminescence dynamics excited at 350 or 430 nm, or on the electronic dynamics measured by femtosecond transient absorption after excitation at 300 nm. We hypothesize that high-energy photons create reactive and emissive charge-separated states in parallel, and that both species are subject to similar electron–hole recombination processes that depend on sample crystallinity. Based on our observations, it can be concluded that the photoluminescence dynamics may be used to evaluate the potential performance of this class of photocatalysts.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...