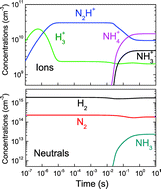
The ion–molecule chemistry of the astronomically relevant H3+, N2H+, and NH4+ ions has been investigated in the weakly ionized cold plasmas formed in glow discharges of H2 with small amounts of nitrogen. The concentrations of neutrals and ions were determined by means of mass spectrometry, and electron temperatures and densities were measured using Langmuir probes. A kinetic model was used for the interpretation of the results. The selection of experimental conditions allowed the generation of ion distributions with different relative weights of the mentioned protonated species and the model calculations showed that the observed ion distributions can be explained by the occurrence of a very efficient H3+ → N2H+ → NH4+ proton transfer chain. The NH4+ ion, which is dominant in most of the cases studied, is ultimately derived from the small amount of NH3 produced at the reactor walls. NH4+ tends to be preponderant in the ion distributions even for NH3 density ratios as low as 1%. Due to the high proton affinity of ammonia, this molecule is readily transformed into NH4+ upon collision with H3+ or N2H+. It is conjectured that these results can be extrapolated to most of the small molecules predominant in the interstellar medium, which also have proton affinities lower than that of NH3. The results support the predictions of astrochemical models indicating that NH4+ could be a preponderant ion in some warm environments like hot cores, where NH3 molecules have desorbed from the grains.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...