Oxygen tracer diffusion along interfaces of strained Y2O3/YSZ multilayers†
Abstract
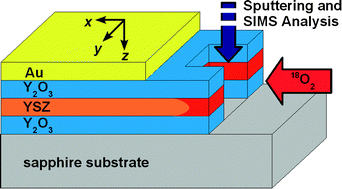
Heterophase boundaries can offer fast transport paths in solid electrolyte materials. In recent studies an enhancement of the ionic conductivity was indeed observed in micro-/nanoscaled Y2O3-stabilised ZrO2 (YSZ) composites and hetero multilayers of thin films. As space charge regions can be neglected due to high charger carrier concentrations, we assume that strain and microstructural changes at the heterophase boundaries are responsible for the observed conductivity effects. In order to obtain independent information on the role of heterophase boundaries for fast transport in strained solid electrolytes, systematic measurements of the 18O-tracer diffusion coefficient in nanoscaled YSZ/Y2O3 multilayers were performed. Multilayer samples were prepared by

 Please wait while we load your content...
Please wait while we load your content...