Gamma-radiation induced formation of chromium oxide nanoparticles from dissolved dichromate
Abstract
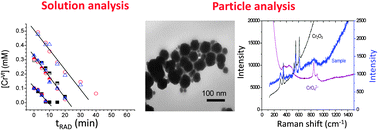
The formation of chromium oxide nanoparticles by gamma radiolysis of CrVI (CrO42− or Cr2O72−) solutions was investigated as a function of pH and initial CrVI concentration by measuring [CrVI], the particle concentration ([CrIII(col)]) and [H2], and by characterizing the particles using TEM, Raman, FTIR and XPS. The results show that CrVI is easily reduced to CrIII by a homogeneous aqueous reaction with ˙eaq−, but, due to the stability of CrIII colloids, the growth of the Cr(OH)3 particles is very slow. As the particles grow the interior of the particle dehydrates to form Cr2O3 while the outer layer remains hydrated. When most of the CrVI that is initially present in the solution is converted to Cr(OH)3 further redox reactions of chromium species occur on the particle surfaces. The redox system reaches a pseudo-equilibrium state due to cyclic reactions of CrIII with ˙OH and H2O2, and reactions of CrVI with ˙eaq− and H2O2. The size distribution of the particles that are formed is controlled by these solution–solid interface reactions.

 Please wait while we load your content...
Please wait while we load your content...