Sodium-doping as a reference to study the influence of intracluster chemistry on the fragmentation of weakly-bound clusters upon vacuum ultraviolet photoionization
Abstract
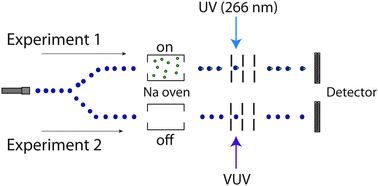
The fragmentation of methanol, water, dimethyl ether, and acetic acid clusters upon photoionization with a single vacuum ultraviolet (VUV) photon of 10.1 eV, 13.3 eV, or 17.5 eV energy is studied with mass spectrometry. The sodium-doping method is used as an independent approximate measure of the original cluster size distribution providing information on the degree of fragmentation upon VUV ionization. The experimental results show strong fragmentation for (CH3)2O and CH3CO2H clusters but minor fragmentation for H2O and CH3OH clusters. The pronounced cluster decay for (CH3)2O and CH3CO2H is explained by additional intracluster chain reactions that occur after the initial fast proton transfer in the ionic clusters, i.e. the decay of (CH3)2O molecules into H2, CO, and CH4 catalyzed by the methoxymethyl radical, and the decay of CH3CO2H molecules into CO2 and CH4 catalyzed by the acetyloxy radical. The absence of equivalent reaction cycles in ionic H2O and CH3OH clusters after the fast proton transfer is consistent with the much less pronounced cluster fragmentation observed upon VUV ionization. The study shows that VUV photoionization even at threshold cannot in general be considered a soft ionization method for weakly-bound clusters, largely because of potential intracluster reaction.

 Please wait while we load your content...
Please wait while we load your content...