Morphology oriented surfactant dependent CoO and reaction time dependent Co3O4 nanocrystals from single synthesis method and their optical and magnetic properties
Abstract
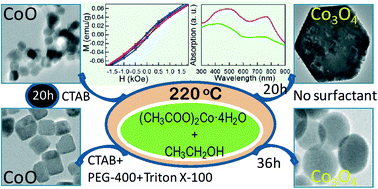
A simple solvothermal strategy has been developed to synthesize highly stable, pure phased CoO and Co3O4 nanoparticles from Co(CH3COO)2·4H2O precursor in ethanol solvent at 220 °C. The various phases of cobalt oxide (cubic CoO and Co3O4) with controlled size and morphologies viz, nanocubes, hexagonal platelets and nanospheres could be obtained via varying only one parameter (either surfactant or reaction time) in this single synthesis method. XRD and HRTEM analyses revealed pure cubic phase CoO and Co3O4 nanoparticles. The formation and shapes of CoO nanoparticles are surfactant dependent, whereas the formation and morphologies of Co3O4 are time dependent. The optical absorbance studies showed two distinct broad absorption peaks and hence two optical band gaps for both CoO and Co3O4 nanoparticles. CoO nanoparticles showed a wide band gap difference of 2.36 eV, while Co3O4 nanoparticles were found to be semiconducting in nature with a 0.6 eV–1 eV band gap difference, which showed characteristic features of CoO and Co3O4 nanoparticles, respectively. CoO nanoparticles showed interesting magnetic behaviour due to intrinsic antiferromagnetic structure and uncompensated surface spins, which was confirmed by VSM and EPR studies.

 Please wait while we load your content...
Please wait while we load your content...