The morphology dependence of cuprous oxide and its photocatalytic properties†
Abstract
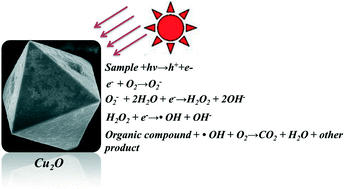
Cuprous oxide (Cu2O) micro-/nanocrystals were synthesized by a simple liquid phase reduction process with copper sulfate as a copper source and glucose monohydrate as a reducer. Detailed investigations of the temperature and feeding manners of the reagents on the morphology and particle size of Cu2O were carried out. X-ray diffraction patterns, morphology observations and ultraviolet–visible diffuse reflection absorption spectra were employed to characterize the crystallinity, morphology and light response. The characterization results gave three extraordinary morphologies: short hexapods, {110} truncated octahedron, and octahedron. The photocatalytic behaviors of the Cu2O crystals were evaluated by monitoring the degradation rate of methyl orange (MO), a target dye. An order for the light photocatalytic activities toward MO was determined: octahedron (diameter 0.172 μm) > short hexapods > octahedron (diameter 5.530 μm) > {110} truncated octahedron crystals. The bewitching structure, controllable morphology and robust photocatalytic performances as well as the easy synthesis mean that the Cu2O crystals are good candidates as light photocatalysts.

 Please wait while we load your content...
Please wait while we load your content...