Cocrystals of fisetin, luteolin and genistein with pyridinecarboxamide coformers: crystal structures, analysis of intermolecular interactions, spectral and thermal characterization†
Abstract
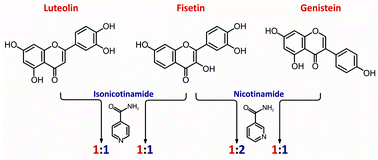
Fisetin, luteolin and genistein, natural polyphenolic compounds of pharmaceutical interest, were combined with nicotinamide and isonicotinamide with an aim to obtain their cocrystals. A screening experiment utilizing solvent-drop grinding was conducted for those combinations. Cocrystalline phases were identified by XRPD and, as far as possible, obtained as single crystals in solution evaporation approach. Five new cocrystals were isolated, characterized by X-ray single-crystal diffraction, FT-Raman spectroscopy, thermal analysis (DSC and TG–DTA), 1H NMR in solution and compared in terms of supramolecular motifs. Reported herein fisetin–nicotinamide (1 : 2) ethanol hemisolvate (FisNam), fisetin–isonicotinamide (1 : 1) (FisInam), two polymorphic forms of luteolin–isonicotinamide (1 : 1) (LutInam, LutInam2) and genistein–nicotinamide (1 : 1) monohydrate (GenNam) cocrystals reveal the presence of an O–H⋯Narom heterosynthon between an O7 hydroxyl moiety of a flavonoid and the pyridyl ring of a coformer. Within those species, mutual orientations of molecules as well as flavonoid–coformer stoichiometry and solvent presence in crystal lattice are factors that imply resulting motif formation and crystal packing.

 Please wait while we load your content...
Please wait while we load your content...