Post-synthetic incorporation of nickel into CPO-27(Mg) to give materials with enhanced permanent porosity†
Abstract
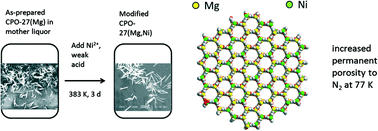
Nickel cations have been introduced into framework sites of the magnesium dioxiterephthalate MOF, CPO-27(Mg), via a procedure involving post-synthetic treatment with an aqueous solution of Ni2+ cations and a weak acid. The final solids have Ni concentrations up to 70 mol% of the total cation content. Selected area EDX analysis, synchrotron X-ray powder diffraction and XPS surface analysis indicate that Ni2+ is distributed throughout the crystals with highest concentration at the external surface. A mechanism involving both crystallisation and predominant isomorphous cation replacement is proposed. The modified solids have much enhanced adsorption capacities following simple thermal evacuation under vacuum than unmodified CPO-27(Mg), particularly for N2 at 77 K, for which uptakes of 17 mmol g−1 are achieved. This is attributed to surface modification that makes the surface more stable to heating under evacuation.
- This article is part of the themed collection: Structural Design of Coordination Polymers

 Please wait while we load your content...
Please wait while we load your content...