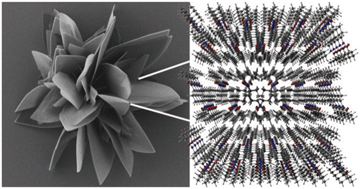
Using a combined approach based on scanning electron microscopy, powder X-ray diffraction as well as 1D and 2D multinuclear solid-state NMR spectroscopy, we were able to determine the morphology and the crystal structures for a set of three supramolecular compounds with different hydrogen bonding motifs, namely N,N′-(cyclohexane-trans-1,4-diyl)bis(2,2-dimethylpropanamide) 1, 1,1′-(cyclohexane-trans-1,4-diyl)bis(3-tert-butylurea) 2 and N1,N4-bis(tert-butylcarbamoyl)cyclohexane-trans-1,4-dicarboxamide 3. Based on a complete signal assignment of the 1D solid-state MAS NMR spectra (1H, 13C, 15N) employing 2D HETCOR experiments and a quantitative evaluation of the corresponding resonances, the content of the asymmetric unit was determined to one half of a molecule. Probing the molecular configuration with 1H–1H double-quantum experiments revealed an intramolecular hydrogen bond for compound 3 while 1 and 2 form exclusively intermolecular H-bonds. Ab initio structure solutions applying real space methods with an included close-contact penalty were carried out for all compounds. The following Rietveld refinements led to excellent wRp-values between 2.5% and 4.1%. Compounds 1 and 2 crystallise isostructurally in the monoclinic space group P21/c exhibiting a pseudo-biaxial hydrogen bond motif. 3 crystallises in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with intermolecular head-to-tail hydrogen bonds connecting the molecules to one-dimensional ribbons. Nevertheless, all compounds grow in a sheet-like morphology with lateral dimensions of several hundred micrometres indicating a fast growth in two dimensions along two of the crystal axes. Since all three molecules possess inversion symmetry cancelling the molecular dipole moment the growth mechanism itself has to be dominantly driven by the formation of hydrogen bond networks.
with intermolecular head-to-tail hydrogen bonds connecting the molecules to one-dimensional ribbons. Nevertheless, all compounds grow in a sheet-like morphology with lateral dimensions of several hundred micrometres indicating a fast growth in two dimensions along two of the crystal axes. Since all three molecules possess inversion symmetry cancelling the molecular dipole moment the growth mechanism itself has to be dominantly driven by the formation of hydrogen bond networks.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with intermolecular head-to-tail hydrogen bonds connecting the molecules to one-dimensional ribbons. Nevertheless, all compounds grow in a sheet-like morphology with lateral dimensions of several hundred micrometres indicating a fast growth in two dimensions along two of the crystal axes. Since all three molecules possess inversion symmetry cancelling the molecular dipole moment the growth mechanism itself has to be dominantly driven by the formation of hydrogen bond networks.
with intermolecular head-to-tail hydrogen bonds connecting the molecules to one-dimensional ribbons. Nevertheless, all compounds grow in a sheet-like morphology with lateral dimensions of several hundred micrometres indicating a fast growth in two dimensions along two of the crystal axes. Since all three molecules possess inversion symmetry cancelling the molecular dipole moment the growth mechanism itself has to be dominantly driven by the formation of hydrogen bond networks.

 Please wait while we load your content...
Please wait while we load your content...