Manganese(ii) and copper(ii) nitrate bis-imidazole coordination polymers: dimensionality and product morphology†
Abstract
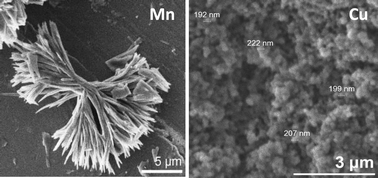
The reaction of a bis-imidazole ligand, N,N′-bis(3-(1-imidazolyl)propyl)-1,4,5,8-naphthalene-tetracarboxylicdiimide (BIPNDI), with either Mn(NO3)2 or Cu(NO3)2 affords coordination polymers of formula {[Mn(BIPNDI)3](NO3)2}∞ or [M(BIPNDI)2(NO3)2]∞ (M = Mn or Cu). Single crystal X-ray diffraction studies confirm the structures of supramolecular isomers of {[Mn(BIPNDI)3](NO3)2}∞ (1,2), both of which contain two-dimensional coordination polymer sheets, and a one-dimensional coordination polymer [Mn(BIPNDI)2(NO3)2]∞ (3). Reaction of either Mn(NO3)2 or Cu(NO3)2 with BIPNDI exploiting rapid precipitation affords sub-micron coordination particles of either rod-shaped (4, Mn) or spherical (5, Cu) morphology depending on the metal salt used. SEM and DLS studies were used to probe the morphology and solution behaviour of the coordination particles. Spectroscopic investigations indicate that the rod-shaped, Mn-based particles comprise two-dimensional coordination polymers in contrast to the spherical, Cu-based particles which are composed of one-dimensional coordination polymers.
- This article is part of the themed collection: Structural Design of Coordination Polymers

 Please wait while we load your content...
Please wait while we load your content...