Anodization strategy to fabricate nanoporous gold for high-sensitivity detection of p-nitrophenol
Abstract
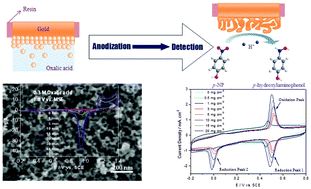
The anodization of a gold electrode in oxalic acid aqueous solution of different concentration has been investigated at different applied potentials, which enables the formation of nanoporous gold (NPG). Electrochemical measurements show that the electrochemically active surface areas of the as-anodized Au samples show an obvious potential, electrolyte concentration and time dependence. The as-prepared NPG samples were used as the working electrode to investigate the redox behavior of p-nitrophenol (p-NP) by cyclic voltammetry (CV). The CV profiles of NPG display a pair of redox peaks, and the peak current densities increase linearly with increasing p-NP concentration. Besides, o-nitrophenol (o-NP) has little influence on the electrochemical detection of p-NP. Through the anodization strategy, NPG electrodes with a high sensitivity and good selectivity can be developed to detect trace p-NP in water.

 Please wait while we load your content...
Please wait while we load your content...