Using 2,5-dimethyl-1,3,4-thiodiazole (L) as ligand, a series of Ag(I), Cu(II) and Ni(II) complexes, namely, [Ag4(μ2-L)6(H2O)2](ClO4)4·2H2O (1), [Ag4(μ2-L)4(μ2-CF3CO2)2(μ3-CF3CO2)2] (2), [Ag2(μ2-L)3(CH3CN)2](AsF6)2 (3), [Ag2(μ2-L)2(μ2-NO3)2]n (4), [Cu(L)2(μ2-NO3)2] (5), [Cu(μ2-L)(μ1,1-N3)3(μ2-CH3COO)]n (6) and [Ni9(μ2-L)6(L)3(μ6-CO3)2(DMF)3(H2O)6(μ3-OH)6]2[Ni9(μ2-L)6(μ6-CO3)2(H2O)12(μ3-OH)6](ClO4)24·9H2O (7) were isolated and their crystal structures determined via single-crystal X-ray diffraction. In 1, six L ligands bridge four neighboring Ag(I) centers forming a tetranuclear cation Ag4 cluster. While in 2, μ3-CF3CO2− anions bridge two pairs of di-nuclear silver(I) clusters forming another type of tetranuclear neutral Ag4 cluster. 3 is a dinuclear Ag(I) complex and 4 is a one-dimensional (1D) chain constructed via dinuclear Ag(I) sub-units, in which μ2-NO3− groups bridge neighboring di-nuclear Ag(I) clusters into a 1D polymeric chain. When Cu(NO3)2 was employed in the reaction system instead of silver(I) salts, a mono-nuclear Cu(II) complex 5 was obtained. When Cu(Ac)2 and NaN3 were further introduced in to the reaction system of 5 instead of Cu(NO3)2, a 1D copper(II) chain 6 constructed with dinuclear copper(II) units was isolated, in which three different types of bridging (μ1,1-azido, acetate and L ligands) can be simultaneously observed. It is interesting that when self-assembly of Ni(ClO4)2·6H2O, L, Na2CO3, and NaN3 in the molar ratio of 4 : 2 : 1 : 8 in CH3OH/DMF/H2O produced well-shaped green crystals of 7. Structural analysis revealed that 7 contains two types of Ni9 clusters with an overall P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c1 non-central symmetry. To our knowledge, 7 represents a unique example of M9L6 and M9L9 cages. The result also reveals that it can be expected that using L ligands as precursors has great potential for constructing these functional materials with intriguing structural motifs such as polynuclear metal clusters. Electrospray ionization mass spectrometry measurements studies for 1–4 demonstrate that in a DMSO solution, complex 1 can still retain the form of a tetranuclear silver(I) cluster. In the solid state, complexes 1–4 exhibit strong fluorescent emission bands, which may be assigned to ligand-to-metal charge transfer. The luminescent properties of these complexes in a DMSO solution varied from blue light to green light at ambient temperature.
c1 non-central symmetry. To our knowledge, 7 represents a unique example of M9L6 and M9L9 cages. The result also reveals that it can be expected that using L ligands as precursors has great potential for constructing these functional materials with intriguing structural motifs such as polynuclear metal clusters. Electrospray ionization mass spectrometry measurements studies for 1–4 demonstrate that in a DMSO solution, complex 1 can still retain the form of a tetranuclear silver(I) cluster. In the solid state, complexes 1–4 exhibit strong fluorescent emission bands, which may be assigned to ligand-to-metal charge transfer. The luminescent properties of these complexes in a DMSO solution varied from blue light to green light at ambient temperature.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c1 non-central symmetry. To our knowledge, 7 represents a unique example of M9L6 and M9L9 cages. The result also reveals that it can be expected that using L
c1 non-central symmetry. To our knowledge, 7 represents a unique example of M9L6 and M9L9 cages. The result also reveals that it can be expected that using L 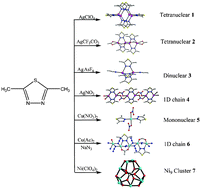

 Please wait while we load your content...
Please wait while we load your content...